Clinical Trial Results for LUTATHERA
In a clinical trial, 229 people with midgut NETs who received LUTATHERA in combination with 30 mg of long-acting octreotide were compared with those who received 60 mg of long-acting octreotide alone.
Longer Progression-Free Survival (PFS) for Patients Treated With LUTATHERA | |
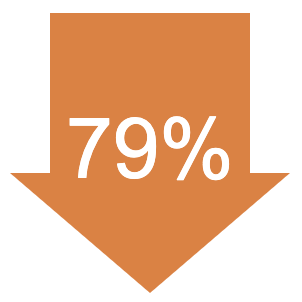
In the LUTATHERA group, the relative risk of the cancer getting worse or death was reduced by 79% compared with people treated with 60 mg of long-acting octreotide alone. | |
| More people treated with LUTATHERA had their tumors shrink compared with people treated with 60 mg of long-acting octreotide alone | |
13% of peoplein the LUTATHERA with 30 mg of long-acting octreotide group
Partial response (tumors shrink)a:
| 4% of peoplein the 60 mg of long-acting octreotide group
Partial response (tumors shrink)a:
0% (0 of 113 people) |
a Tumors shrink to ≥30% from baseline.
b Tumors disappear and cancerous lymph nodes shrink to <10 mm. The disappearance of any measurable tumors does not necessarily mean that the cancer is completely gone.