LUTATHERA Is Believed to Work Differently From Most Other Cancer Treatments
LUTATHERA is the first and only approved radioligand therapy (also known as RLT) for GEP-NET, a medicine from a class of drugs called peptide receptor radionuclide therapy (also known as PRRT).
LUTATHERA is believed to work differently from other cancer medicines, with a 2-part approach that specifically targets and enters the cells that have somatostatin receptors, releasing energy in the form of radiation that damages them and nearby cells.
In other words, LUTATHERA is a “key” that connects with the “lock” (cells containing somatostatin receptors).
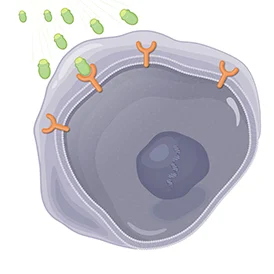
1. Attaches to Target Cells | |
LUTATHERA is designed to contain a tumor-targeting part that attaches to cells with somatostatin receptors, including GEP-NET cancer cells. | |
Once it finds these cells, LUTATHERA is designed to bind to somatostatin receptors located on the outside of the cells. | |
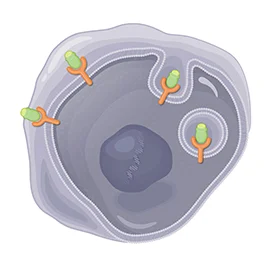
2. Enters Into Cells | |
After LUTATHERA binds to the somatostatin receptors, it is designed to enter into the cell. | |
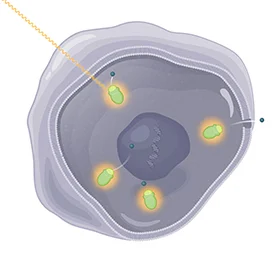
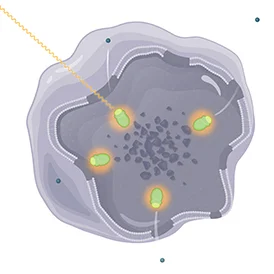
Finally, LUTATHERA is designed to deliver the radiation that causes damage to the targeted cells with the somatostatin receptor and neighboring cells. | |